

Have you ever wondered how gasoline is efficiently produced from crude oil, or how certain plastics and detergents are made? Behind many of these large-scale industrial processes lies a powerful and often unsung hero: the zeolite catalyst.
At its core, a catalyst is a substance that speeds up a chemical reaction without being consumed itself. Think of it as a master facilitator. Among them, zeolites stand out for their incredible precision and efficiency.
What Exactly is a Zeolite?
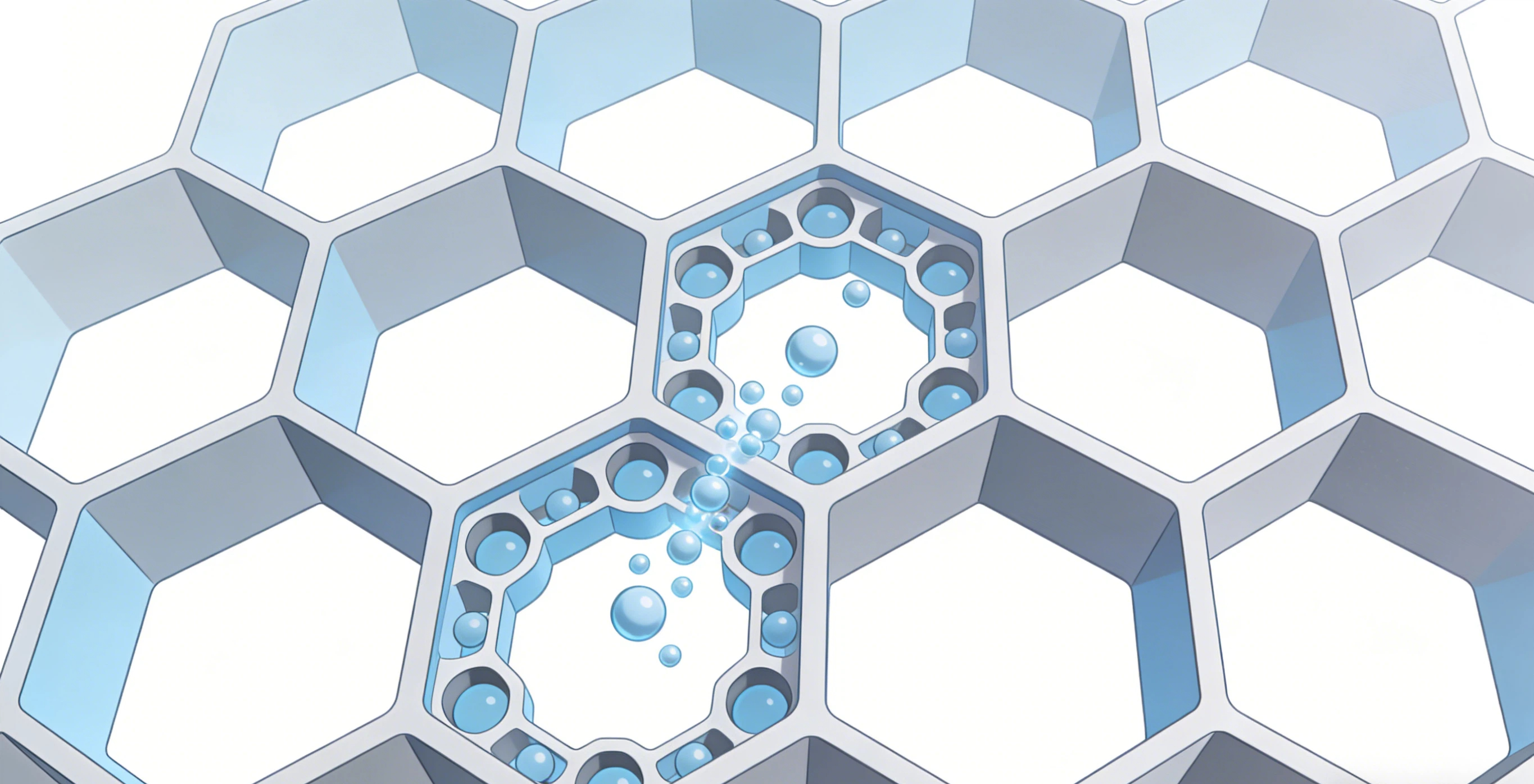
Zeolites are crystalline, porous materials primarily made of silicon, aluminum, and oxygen. Their name comes from Greek words meaning "boiling stone," due to their ability to release trapped water when heated. But the real magic is in their structure.
Imagine a rigid, honeycomb-like framework at the atomic scale. This framework creates a vast network of uniform, molecular-sized channels and cages. This is why they are often called "molecular sieves." They can literally screen molecules based on size and shape, allowing only certain ones to enter and react within their pores.
Why Are Zeolites Such Great Catalysts?
Their power comes from a combination of three key features:
High Surface Area & Porosity: The intricate pore system provides a massive internal surface area—often hundreds of square meters per gram—where reactions can take place.
Acidic Sites: When aluminum (Al³⁺) substitutes for silicon (Si⁴⁺) in the framework, it creates a localized negative charge. This is balanced by positively charged ions (like H⁺), creating powerful Brønsted acid sites. These acid sites are crucial for breaking and forming bonds in hydrocarbon molecules, driving reactions like cracking and isomerization.
Shape-Selectivity: This is their superstar trait. Zeolites can control chemical reactions based on molecular geometry:
Reactant Selectivity: Only molecules small enough to enter the pores can react.
Transition State Selectivity: Bulky intermediate compounds formed during a reaction are restricted, steering the process toward desired, smaller products.
Product Selectivity: Only products with a specific shape can exit the pores, preventing the formation of unwanted larger byproducts.
Where Do We Use Zeolite Catalysts?
You likely encounter products made with their help every day:
Oil Refining (Fluid Catalytic Cracking - FCC): This is the largest application. Zeolite catalysts (like fantastic Y-type) "crack" heavy, long-chain petroleum fractions into valuable, lighter products such as gasoline, diesel, and propylene.
Petrochemicals: They are essential in producing paraxylene (a precursor for polyester plastics and fabrics), and in isomerization processes to boost octane ratings in fuels.
Fine Chemicals & Intermediates: Zeolites enable cleaner, more selective synthesis routes for various chemical intermediates.
Environmental Protection: They are key in catalytic converters for diesel engines (SCR catalysts) to reduce harmful NOx emissions by converting them into harmless nitrogen and water. They are also used in air and water purification.
Looking Ahead
Research continues to advance the field. Scientists are developing novel zeolite structures, synthesizing nanosized zeolites for faster reactions, and fine-tuning their acidity to create even more selective and energy-efficient catalysts for sustainable chemistry.